Molecular and elemental mapping of nanomaterials in vitro and in vivo. Dosimetry of nanomaterials (NanoReg)
A common European Approach to the Regulatory Testing of Nanomaterials (NANoREG)
Launched in March 2013. Duration 3.5 years
The NANoREG project is supported by European Commission in the framework of FP7 Theme-NMP.2012.1.3-3 (Nanosciences, Nanotechnologies, Materials and New Product Technologies).
Grant Agreement Number: 310584
The innovative and economic potential of Manufactured Nano Materials (MNMs) is threatened by a limited understanding of the related EHS issues. While toxicity data is continuously becoming available, the relevance to regulators is often unclear or unproven. The shrinking time to market of new MNM drives the need for urgent action by regulators. NANoREG is the first FP7 project to deliver the answers needed by regulators and legislators on EHS by linking them to a scientific evaluation of data and test methods. Based on questions and requirements supplied by regulators and legislators, NANoREG will: (i) provide answers and solutions from existing data, complemented with new knowledge, (ii) provide a tool box of relevant instruments for risk assessment, characterisation, toxicity testing and exposure measurements of MNMs, (iii) develop, for the long term, new testing strategies adapted to innovation requirements, (iv) establish a close collaboration among authorities, industry and science leading to efficient and practically applicable risk management approaches for MNMs and products containing MNMs. The interdisciplinary approach involving the three main stakeholders (Regulation, Industry and Science) will significantly contribute to reducing the risks from MNMs in industrial and consumer products. NANoREG starts by analysing existing knowledge (from WPMN-, FP- and other projects). This is combined with a synthesis of the needs of the authorities and new knowledge covering the identified gaps, used to fill the validated NANoREG tool box and data base, conform with ECHA’s IUCLID DB structure. To answer regulatory questions and needs NANoREG will set up the liaisons with the regulation and legislation authorities in the NANoREG partner countries, establish and intensify the liaisons with selected industries and new enterprises, and develop liaisons to global standardization and regulation institutions in countries like USA, Canada, Australia, Japan, and Russia.
The NANoREG project is carried out by a consortium of 59 partners comprising state institutes, universities and legislation authorities as well as industrial partners. It is a follow-on project after HINAMOX. The Leipzig group is involved in two work packages „Biokinetics and toxicity testing in vivo“ and „Advancement of Regulatory Risk Assessment and Testing“.
The following issues will be addressed in these work packages:
- 1. Quantification of effective or genuine concentration of nanomaterials (NM) inside cells and tissues;
- 2. Knowledge of Exposure-Genuine Dose and Genuine Dose-Response relationships;
- 3. Validation of in vitro tests concerning their significance for in vivo results;
- 4. Distribution and localization of NM in organs, tissues and cells down to the subcellular level.
- 5. Characterization of real appearance of NMs in the biological environment.
In the framework of NANoREG the Leipzig group takes part in a two year study on inhalative long term particle distribution, toxicity, and carcinogenicity (OECD 453) of CeO2 particles in lung tissue. The partners of this study are the Federal Institute for Risk Assessment (Berlin), the Fraunhofer Institute for Toxicology and Experimental Medicine (Hannover) and BASF (Ludwigshafen). As methods Ion Beam Microscopy (IBM), Confocal Raman Microspectroscopy (CRM), Time of Flight Secondary Ion Mass Spectroscopy (ToF-SIMS) and extended Histopathology will be used.
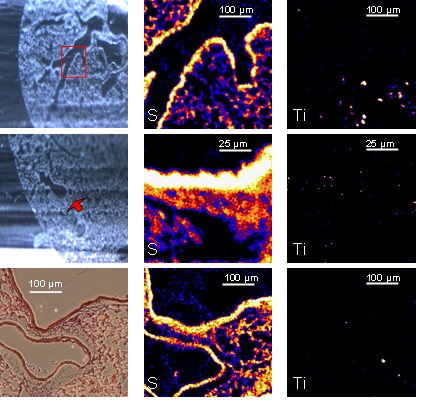
Examples of imaging at tissue level with Ion Beam Microscopy (IBM)
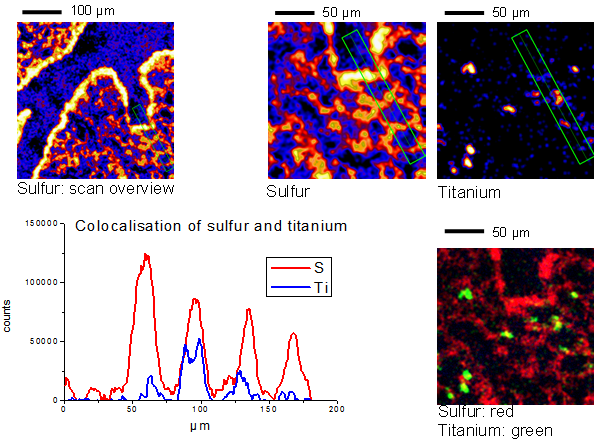
Fig. 2. Colocalization of TiO2 NMs and sulfur in lung alveolar tissue. A specific region containing three alveoli was selected and inspected at higher resolution (upper three images). The lower left graph provides the colocalization along the selected rectangular region of interest. Bottom right image: Overlay of sulfur and TiO2 NMs distribution.
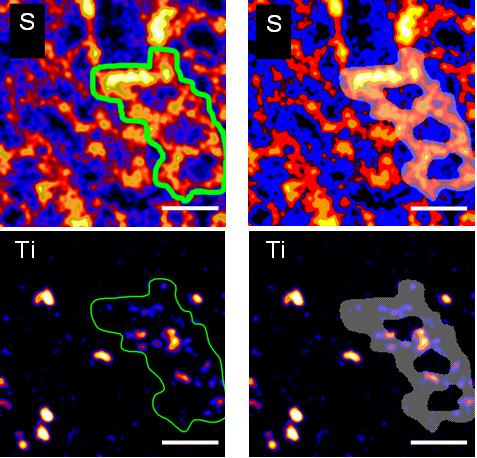
| P | S | K | Ca | Ti | Fe | Zn |
| 1208 | 2259 | 345 | 228 | 240 | 102 | 94 |
Fig. 3. Quantification of TiO2 NMs in the selected alveolar region from Fig. 4. The table provides the elemental concentrations given in ppm in the selected region. The bars represent 50 µm.