Fields of Research
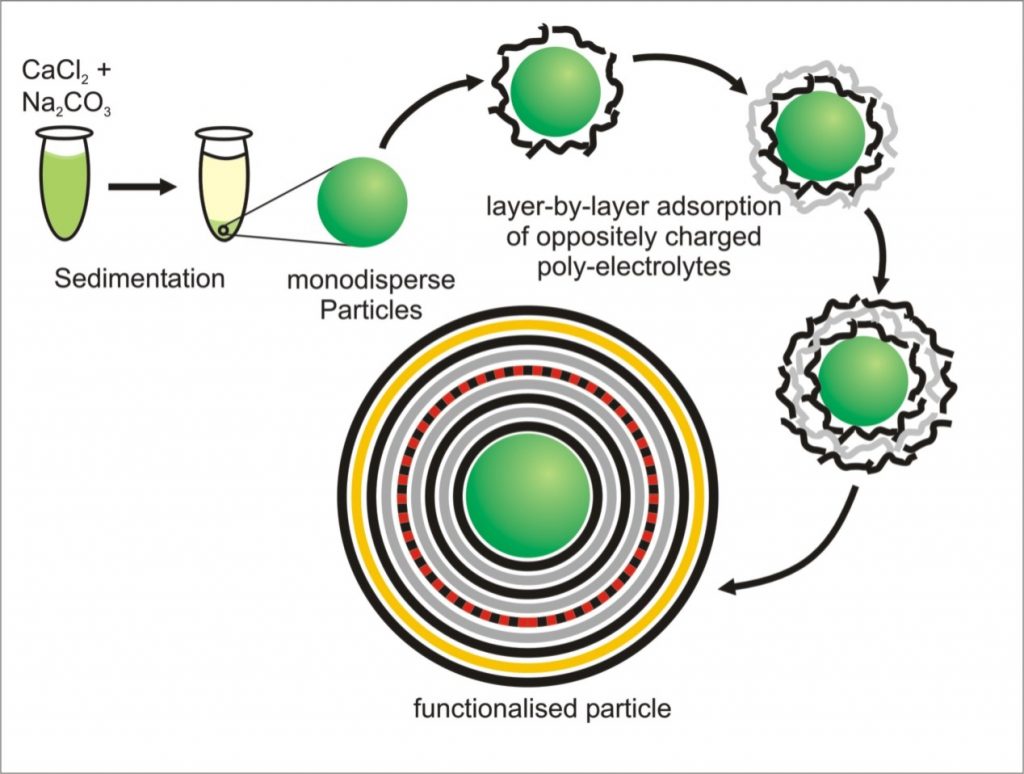
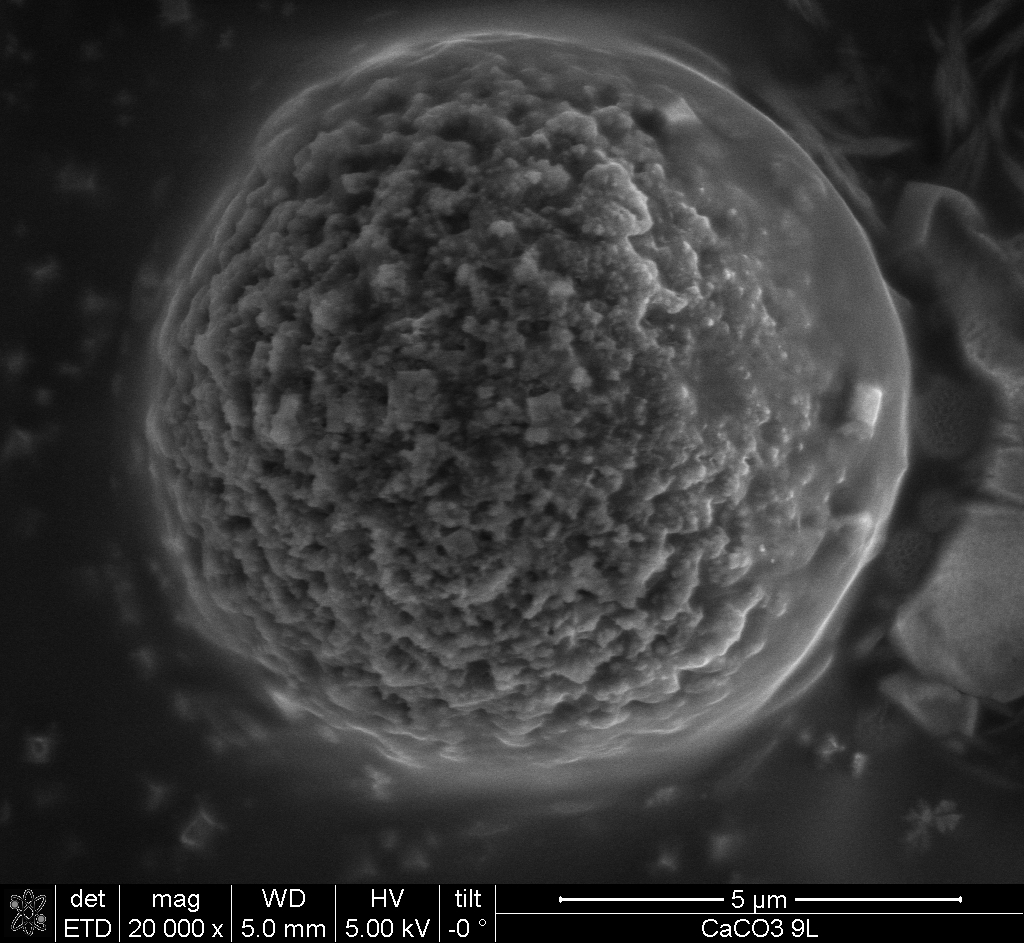
After the introduction of Layer-by-Layer assembling of oppositely charged polyelectrolytes on flat and colloidal templates in the nineties, this technique became more and more interesting for biomedical applications with the design of multifuctional carrier systems. Colloidal microcarrier systems show a great potential in drug delivery due to their modular contruction: several active agents can be embedded into a biocompatible polymer multilayer or core with the option of additional carrier functionalization such as for cell surface mimicking or by the integration of sensors. Thus, microcarriers combine several important advantages in transport and delivery of active agents into living cells and tissue compared to current therapy concepts:
- biocompatible and biodegradable multilayer material as well as the core dissolution after multilayer assembling provide high biocompatibility reducing off-target effects,
- the wide choice of multilayer material, the biopolymer layer number and the layer depth of the assembled active agent(s) provide a time-controlled release of the agents within the cell,
- different active agents can be assembled into different layer depths without interference, facilitating a time-delayed application of the agents,
- additional surface modifications provide a targeted or enhanced uptake by cells, and
- agents can be applied in a defined dosage by varying their amounts within the multilayer or core.
Our group is working on the characterization and improvement of addressing, uptake and intracellular processing of the carriers as well as on the development of microcarrier systems for specific applications.
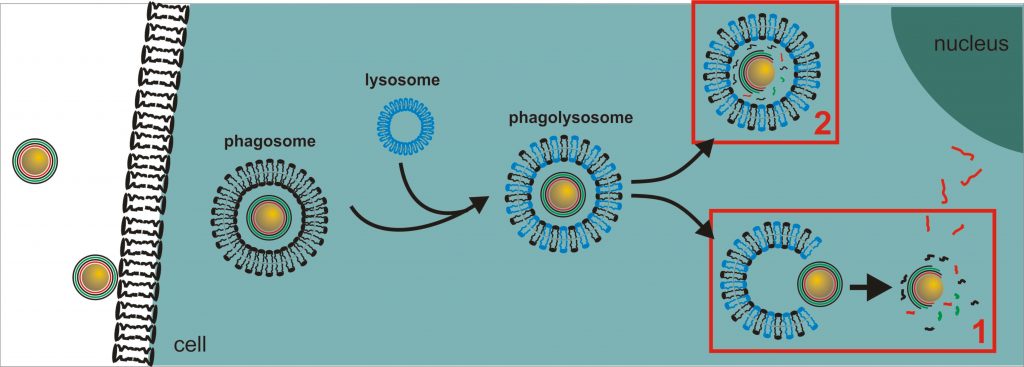
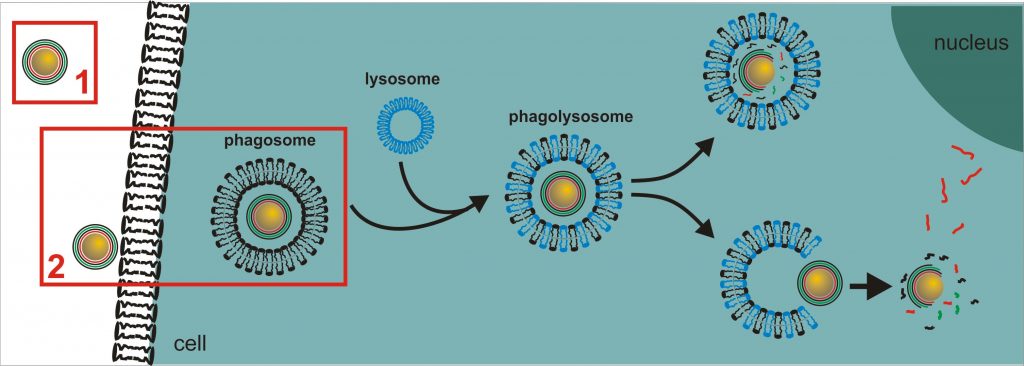
Development and design of colloidal microcarriers as drug delivery system require a profound knowledge of the carrier adsorption on cell membranes, endo-/phagosomal uptake by cells, processing within endo-/phagolysosomes and the potential release of the carriers within the targeted cell compartment, such as endo-/phagolysosome or cytoplasm, to deliver the agents with full activity but only low carrier-induced negative effects on the cell.
Focus on:
- Biopolymers with specific properties for multilayer disassembly in
- phagolycosomes
- cytoplasm
- LbL particles vs. LbL capsules
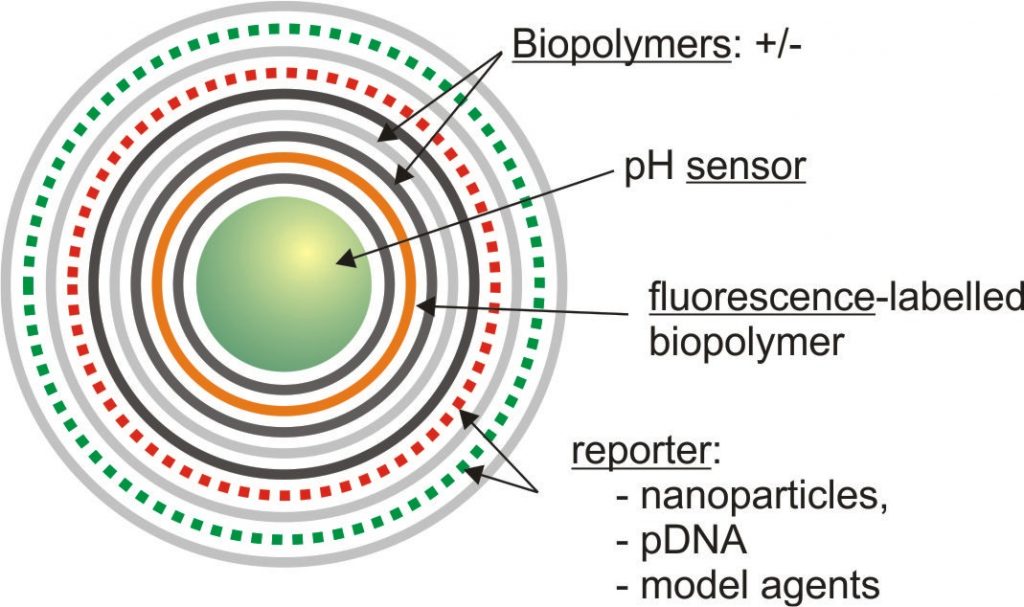
- Integration of and for carrier tracking and quantification during all stages of processing using
- pH sensors
- plasmid-DNA coded for fluorescent proteins
- nanoparticles
- fluorescence labelled polymers
- Development and integration of to facilitate specific demands to
- protect active agents
- gain access to cytoplasm
- support targeted multilayer disassembly
One the one hand, we focus on the carrier transport into cytoplasm, the cell compartment most frequently targeted for drug delivery. After uptake, the endo-/phagolysosomal barrier has to be overcome, a general problem in efficient drug delivery, since the degrading potential of enzymes may reduce the carrier efficacy.
However, to design an efficient drug delivery system, sensor components are required to assess and quantify carrier release rate, time frame and remaining activity of the delivered agent. Recent investigations use multilayer-integrated plasmid DNAs (pEGFP, pDsRed) as reporters which are coded for fluorescent proteins (1,2) to prove the carrier efficacy. Successful transport and delivery into the cytoplasm of cells can thus be visualized by confocal microscopy (CLSM) and flow cytometry (FCM) after protein expression(1). The additional integration of pH sensors into the core as well as fluorescence labelled polymers facilitate the quantification of the carrier processing from endo-/ phagolysosome to cytoplasm(2-4). The following time-dependent multilayer decomposition within the cytoplasm can be observed e.g. by using magnetite nanoparticles as reporters which are integrated into the multilayer(5). Their release by means of a step-by-step multilayer disassembly is then detectable by means of ion beam techniques (as Proton-Induced X-ray Emission (PIXE)).
Key papers:
Reibetanz, U., Claus, C., Typlt, E., Hofmann, J., Donath, E. Defoliation and Plasmid Delivery with Layer-by-Layer Coated Colloids. Macromol. Biosci. 6 (2006) 153-160. [DOI, PubMed]
Reibetanz U, Chen MHA, Mutukumaraswamy S, Liaw ZY, Oh BHL, Venkatraman S, Donath E, Neu B. Colloidal DNA Carriers for Direct Localization in Cell Compartments by pH Sensoring. Biomacromolecules 11 (2010) 1779-1784. [http://dx.doi.org/10.1021/bm100237rPubMed]
Reibetanz, U., Halozan, D., Brumen, M., Donath, E. Flow Cytometry of HEK 293T Cells Interacting with Polyelectrolyte Multilayer Capsules Containing Fluorescein-Labeled Poly(acrylic acid) as a pH Sensor. Biomacromolecules 8 (2007) 1927-1933. [DOI, PubMed]
Reibetanz U, Chen M H A, Mutukumaraswamy S, Liaw Z Y, Oh B H L, Donath E, Neu B. Functionalization of Calcium Carbonate Microparticles as a Combined Sensor and Transport Sytem for Active Agents in Cells. J. Biomat. Sci.-Polym. E. 22 (2011) 1845–1859. [DOI]
Reibetanz U, Jankuhn S. Magnetite Nanoparticles as Reporters for Microcarrier Processing in Cell Cytoplasm. Nucl. Instrum. Meth. Phys. Res. B 269 (2010) 2281-2285. [DOI]
Surface Modification
The modular LbL construction allows a surface functionalization of the carriers independent of their internal design. Such a modification can be used to facilitate specific or enhanced uptake but may also be applied to protect the inner structure from opsonization or early degradation during carrier transport.
Focus on:
- Lipid membrane
- Integration of functional lipids
- Combination of different surface functionalities
- carrier shielding and protection
- Enhanced uptake
- Cell-penetrating peptides
- Virus-like particles
- Specific uptake
- Antibodies
1. Shielding of the Microcarrier's Inner Structure and Functionalization towards Specific Uptake: Lipid Membrane and Antibodies
The targeted addressing of specific cells is another important issue in microcarrier functionalization in order to transport defined amounts of active agents as well as to avoid unintended side-effects in other cells within the tissue. The objectives here are focused on the specific uptake of LbL carriers by cells, with emphasis placed on an enhanced biocompatibility and minimization of non-specific interactions with biological systems. Biocompatibility and protective function can be obtained by a homogeneously and tightly assembled phospholipid membrane as a supported lipid layer on top of the underlying biopolymer multilayer(1). The integration of a functional lipid (e.g. phosphatidylethanolamine, PE) equipped with polyethylene glycol (PEG) enhances the shielding properties of the carrier. Biotinylated PEG can be used for the coupling of additional functional molecules via streptavidin/biotin binding(2). Thus, e.g. different antibodies can be assembled without disturbing the construction of the inner structure, since homogeneity and tightness of the lipid membrane depend strongly on the polymer material, lipid components, and assembly conditions(1).
2a. Fast Uptake of Microcarriers by Cells: Cell-Penetrating Peptides
Another aim is the enhancement of cellular uptake, for example by faster carrier incorporation by cells. A fast uptake is very important for drug delivery systems, since early and unintended carrer degradation in extracellular space may thus be reduced. Cell penetrating peptides, assembled as outermost layer, offer a good opportunity to enhance the uptake of carriers into cells(3).
2b. Enhanced Cell Entry of Microcarriers: Virus-like Particles
Enhanced uptake can also be initialized by virus-like particles assembled onto the LbL-carrier’s surface. The fusion of enveloped virus-like particles with an underlying lipid membrane at low pH ensures embedding of the capsid and presentation of the fusion proteins on the overall surface(4,5). Although only shown with Rubella-like particles, the universal assembly technique allows the application of a wide variety of viral systems.
Key papers:
(4) Fischlechner, M., Zschörnig, O., Hofmann, J., Donath, E. Engineering Virus Functionalities on Colloidal Polyelectrolyte Lipid Composites. Angew Chem Int Ed Engl. 44 (2005) 2892-2895. [DOI]
(5) Fischlechner, M., Reibetanz, U., Zaulig, M., Enderlein, D., Romanova, J., Leporatti, S., Moya, S., Donath, E. Fusion of Enveloped Virus Nanoparticles with Polyelectrolyte-Supported Lipid Membranes for the Design of Bio/Nonbio Interfaces. Nano Lett. 7 (2007) 3540-3546. [DOI]
Funding:
- German Research Foundation (DFG, RE 2681/2-1)
- PhD scholarship: ESF, European Union and the Free State of Saxony, supported by DFG graduate school 185 „Leipzig School of Natural Sciences Building with Molecules and Nono-objects“ (BuildMoNa) (M. Göse)
- Scholarship: Formel.1 program, Medical Faculty, Universität Leipzig
Specific Application of LbL Microcarriers in Drug Delivery
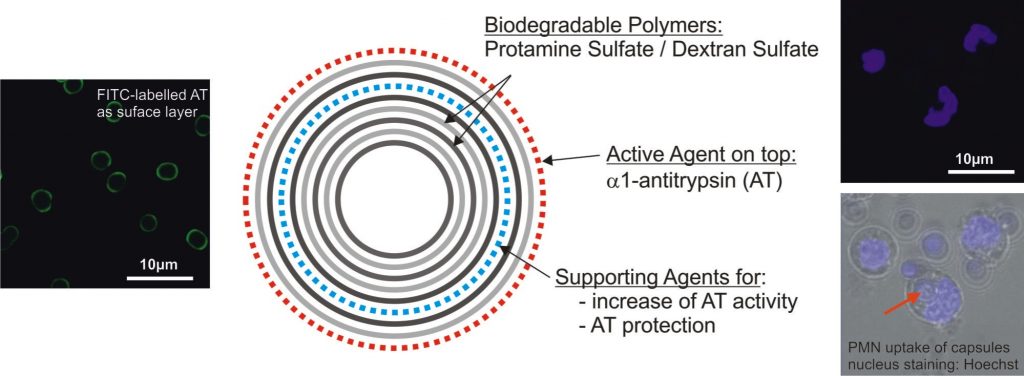
Another objective is the development of specific drug delivery systems such as a combined transport and sensor system for anti-inflammatory substances (AIS). The layer-by-layer coated carriers are intended to transport and release a defined amount of AIS into the inflamed tissue in order to terminate chronically proceeding processes and to facilitate the regeneration of tissue homeostasis. The simultaneous release of AIS into the phagolysosomes of polymorphonuclear leukocytes (PMNs) as well as into the extracellular space is a new approach for an effective inhibition of pro-inflammatory substances originating from these cells and macrophages. In particular, anti-protease a1-antitrypsin was transported by LbL microcarriers to inhibit the protease elastase at its place of origin.
Focus on:
- Design of specific LbL-based transport systems for AIS
- Defined amounts of a1-antitrypsin as multilayer constituent
- parallel integration of additional substances and sensors
- LbL particles and LbL capsules
- Carrier/cell interaction: PMNs
- Fast carrier uptake
- Fast multilayer disassembly within phagolysosomes and AIS release
- Efficient intracellular elastase inhibition
- Parallel extracellular inhibition of already released elastase
- Intra- and extracellular elastase inhibition combined in one
carrier - Negligible carrier effect on cell vitality
- Carrier/cell interaction: macrophages
- Negligible carrier effect on cell vitality
- No carrier-induced pro-inflammatory signaling
.
1. Carrier Design
For an application in PMNs, LbL microcarriers have to be built up by an internally easily degradable multilayer and facilitate the integration of a defined amount of AIS.
The amount and activity of a1-antitrypsin (AT) essential for the inhibition of intracellular elastase can be determined in concentration-dependent studies of AT/elastase interaction under phagolysosomal and extracellular conditions(1) by means of spectrophotometry. Under ideal conditions, the assembly of AT as only one outer layer of the biopolymer multilayer and the application of one carrier per cell is sufficient to efficiently inhibit intracellular elastase(2). Extending the experiments towards capsules, coating conditions of AT have to be adapted to the dissolution procedure of the core material, since this treatment may influence the amount and activity of AT as previously assembled multilayer constituent(3).
Key Publications:
- Schönberg, M., Reibetanz, U., Rathmann, S., Leßig, J. Maintenance of α1-antitrypsin activity by means of co-application of hypochlorous acid scavengers in vitro and the supernatant of polymorphonuclear leukocytes – As a basis for a new drug delivery approach. Biomatter 2 (2012) 1-13. [DOI]
- Reibetanz, U., Schönberg, M., Rathmann, S., Strehlow, V., Göse, M., Leßig, J. Inhibition of Human Neutrophil Elastase by Alpha1-Antitrypsin Functionalized Colloidal Microcarriers. ACS Nano 6 (2012) 6325-5336. [DOI]
- Strehlow, V., Leßig, J., Göse, M., Reibetanz, U. Development of LbL-biopolymer capsules as delivery system for the multilayer-assembled anti-inflammatory substance α1-antitrypsin. J. Mater. Chem B. 1 (2013), 3633–3643 [DOI]
2. Carrier/PMN Interaction
The functionalized microcarriers are then applied as drug transport and delivery systems for AT addressing PMNs (isolated from blood) and PMN-like differentiated U 937(4). Compared to other applications, the intracellular target here is already the phagolysosome, since after phagocytosis a fusion of carrier-containing phagosomes and enzyme-containing granules and lysosomes occurs followed by enzyme triggered multilayer disassembly, release of anti-protease AT and subsequent elastase inhibition. However, those carriers have to undergo a very fast uptake in order to disassemble and release the agents during the very short PMN’s life time of about 24 h(5).
Using the same construction, extracellular remaining AT-functionalized carriers are capable of inhibiting already released elastase in parallel to the internalized carriers. The non-internalized carriers immobilize the protease on their surfaces by AT/elastase complexation(6).
Additionally to their transport abilities, the LbL carriers themselves are intended to have only low negative influence on cell viability, i.e. on apoptosis or necrosis characteristics. In this context, AT functionalized LbL carriers even induce a positive effect on PMN’s vitality by delaying apoptosis(7).
In parallel to LbL-mediated AT transport, the efficiency of a cell-penetrating peptide (CPP)-mediated AT administration was investigated comparing advantages and disadvantages of both carrier systems (8).
Key Publications:
- Leßig J, Neu B, Glander H-J, Arnhold J, Reibetanz U. Phagocytotic Competence of Differentiated U937 Cells for Colloidal Drug Delivery Systems in Immune Cells. Inflammation 34 (2011) 99-110. [DOI, PubMed].
- Rathmann, S., Schönberg, M., Leßig, J., Reibetanz, U. Interaction, Uptake and Processing of LBL-coated Microcarriers by PMNs. Cytom. Part A 79(2011) 979-989. [DOI]
- Reibetanz, U., Schönberg, M., Rathmann, S., Strehlow, V., Göse, M., Leßig, J. Inhibition of Human Neutrophil Elastase by Alpha1-Antitrypsin Functionalized Colloidal Microcarriers. ACS Nano 6 (2012) 6325-5336. [DOI]
- Fichtner, M., Claus, C., Lessig-Owlanj, J., Arnhold, J., Reibetanz, U. The Application of LbL-Microcarriers for the Treatment of Chronic Inflammation: Monitoring the Impact of LbL-Microcarriers on Cell Viability. Macromol. Biosci. (2014), in press [DOI]
- Leßig, J., Reibetanz, U., Schönberg, M., Neundorf, I. Efficient Inhibition of Human Leukocytic Elastase by means of Alpha1-Antitrypsin/Peptide Complexes. Cytometry: Part A 83 (2013) 461-471. [DOI]

3. Carrier/Macrophage Interaction
Beside the carrier interaction with PMNs, the role of macrophages regarding the clearance of apoptotic or necrotic PMNs and microcarriers has to be considered. In particular, the carriers are not intended to induce pro-inflammatory signalling of macrophages. Experiments regarding cell interaction with colloidal microparticles as well as with microcapsules show only a very low negative influence on the cells(9,10).
Key Publications:
- Leßig, J., Neu., B., Reibetanz, U. Influence of Layer-by-Layer (LbL) Assembled CaCO3-Carriers on Macrophage Signaling Cascades. Biomacromolecules 12 (2011) 105-115. [PubMed]
- Strehlow, V., Leßig, J., Göse, M., Reibetanz, U. Development of LbL-biopolymer capsules as delivery system for the multilayer-assembled anti-inflammatory substance α1-antitrypsin. J. Mater. Chem B. 1 (2013), 3633–3643 [DOI]
Funding:
- Translational Center for Regenerative Medicine, Leipzig, German Federal Ministry of Education and Research (BMBF, PtJ-Bio, 0313909); Co-Investigator: Dr. J. Lessig
- German Research Foundation (DFG, RE 2681/2-1)
- German Research Foundation (DFG, RE 2681/2-2)
.