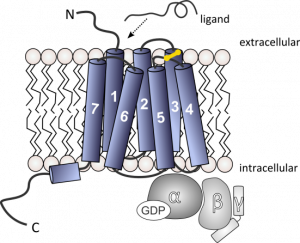
Schematic illustration of a GPCR bound to the heterotrimeric G-protein complex. Ligand binding on the extracellular side induces the activation of the receptor which is accompanied by a conformational change within the transmembrane region of the receptor. This results in an interaction with the heterotrimeric G-protein on the intracellular side and the replacement of GDP by GTP within the Gα subunit. Understanding the structure and dynamics of this complex will support the treatment of numerous diseases.
G protein-coupled receptors (GPCRs) and the interaction with their natural ligands are essential in various signal transduction pathways and consequently represent an important and promising target for pharmaceutical research. For the structure-based drug development, atomistically resolved conformational data represent a prerequisite which can be provided by NMR and EPR spectroscopy.
Our target GPCRs are the peptide-binding Y receptors as well as the growth hormone secretagogue receptor, typical class A GPCRs. The aim of our research is to characterize their structure and function with respect to the dynamics of the receptors and the binding behavior of their ligands. To this end, the GPCRs are expressed either in a cell-free expression system or in E. coli as inclusion bodies in high density fermentation processes using defined media. Subsequently, the receptors are isolated, solubilized in detergent, purified in vitro and finally reconstituted into phospholipid bilayers. With the established protocols, it is possible to obtain sufficient amounts of labelled protein that can be employed in NMR and other biophysical studies.
Methods used in the Huster lab related to GPCR research:
– molecular biology (cloning, mutagenesis)
– fermentation and expression in E. coli
– cell-free expression
– inclusion body preparation and affinity chromatography for protein purification
– reconstitution in lipid bicelles
– fluorescence polarization assay
– NMR spectroscopy both in solution and in the solid state
– EPR spectroscopy
Key Papers:
- P. Schmidt, H. Scheidt, L. Thomas, P. Müller, D. Huster. The G protein-coupled neuropeptide Y receptor type 2 is highly dynamic in lipid membranes as revealed by solid-state NMR spectroscopy. Chem. Eur. J. 20 (2014) 4986-4992.
- G. Vortmeier, S.H. DeLuca, C. Cholet, H.A. Scheidt, A.G. Beck-Sickinger, J. Meiler, D. Huster. Integrating solid state NMR and computational modeling to investigate the structure and dynamics of membrane-associated ghrelin. PLoS One (2015) e0122444.
- A. Kaiser, P. Müller, T. Zellmann, H.A. Scheidt, M. Bosse, R. Meier, J. Meiler, D. Huster, A.G. Beck-Sickinger, P. Schmidt. Unwinding of the C-terminal residues of the neuropeptide Y is critical for the Y2 receptor binding and activation. Angew. Chem.Int. Ed. 54 (2015) 7446-7449.
- S. Schrottke, G. Vortmeier, S. Els-Heindl, M. Bosse, P. Schmidt, H.A. Scheidt, A.G. Beck-Sickinger, D. Huster. Expression, Functional Characterization, and Solid-State NMR Investigation of the G Protein-Coupled GHS Receptor in Bilayer Membranes. Sci. Rep. 7 (2017).
- P. Schmidt, B.J. Bender, A. Kaiser, K. Gulati, H.A Scheidt, H.E. Hamm, J. Meiler, A.G. Beck-Sickinger, D. Huster. Improved in vitro folding of the Y2 G protein-coupled receptor into bicelles. Front. Mol. Biosci. 4 (2018) e100.
- Z. Yang, S. Han, M. Keller, A. Kaiser, B.J. Bender, M. Bosse, K. Burkert, L. M. Kögler, D. Wifling, G. Bernhardt, N. Plank, T. Littmann, P. Schmidt, C. Yi, B. Li, S. Ye, R. Zhang, B. Xu, D. Larhammar, R.C. Stevens, D. Huster, J. Meiler, Q. Zhao, A.G. Beck-Sickinger, A. Buschauer, B. Wu. Structural basis of ligand binding modes of neuropeptide Y Y1 receptor. Nature 556 (2018) 520-524.
- B.J. Bender, G. Vortmeier, S. Ernicke, M. Bosse, A. Kaiser, S. Els-Heindl, U. Krug, A.G. Beck-Sickinger, J. Meiler, D. Huster. Structural model of ghrelin bound to its G protein-coupled receptor. Structure 27 (2019) 537-544e4.
Team:
Dr. Marta Gozzi
Cindy Montag
Jeannette Laugwitz
Emelyne Pacull
Dr. Peter Schmidt
Matthias Voitel
Collaborations:
- Prof. Dr. Annette G. Beck-Sickinger, Institute of Biochemistry, Leipzig University
- Prof. Dr. Jens Meiler, Center for Structural Biology, Vanderbilt University, Nashville, USA
- Prof. Dr. Heidi Hamm, Department of Pharmacology, Vanderbilt University, Nashville, USA
- Prof. Dr. Vsevolod Gurevich, Department of Pharmacology, Vanderbilt University, Nashville, USA
- Prof. Dr. Hassane Mchaourab, Molecular Physiology & Biophysics, Vanderbilt University, Nashville, USA